The need for Pharmacovilance (PV)

Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem.
Before a medicine is authorized for use, evidence of its safety and efficacy is limited to the results from clinical trials, where patients are selected carefully and followed up very closely under controlled conditions. This means that at the time of a medicine’s authorization, it has been tested in a relatively small number of selected patients for a limited length of time. After authorization the medicine may be used in a large number of patients, for a long period of time and with other medicines. Certain side effects may emerge in such circumstances.
It is therefore essential that the safety of all medicines is monitored throughout their use in healthcare practice.
Viet Phap is one of the first Vietnamese companies having Pharmacovigilance (PV) department. We have passed some on-site PV audits from big EU partners and multinational corporation.
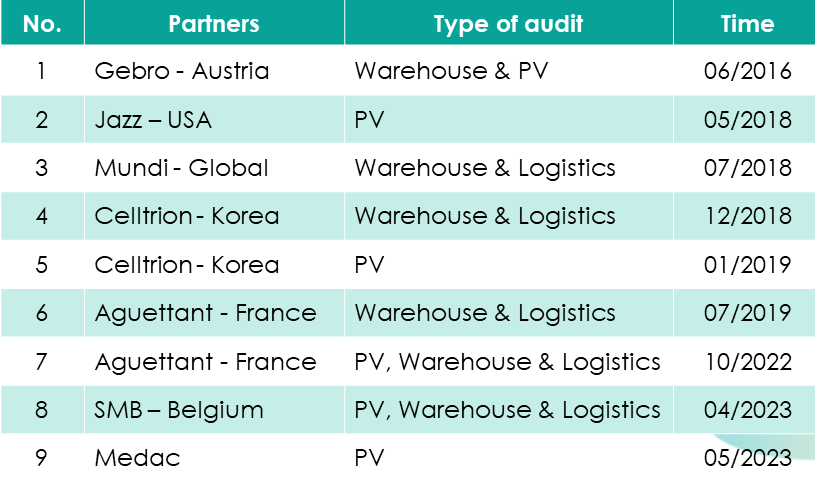
If you have any information in adverse reaction of our drugs, please contact us with the following email.
Viet Phap – Pharmacovigilance Department
20th Floor, ICON 4 Tower, 243A De La Thanh street, Lang Thuong ward, Dong Da district, Ha Noi, Vietnam
Email: drugsafety@vietphapco.com
